
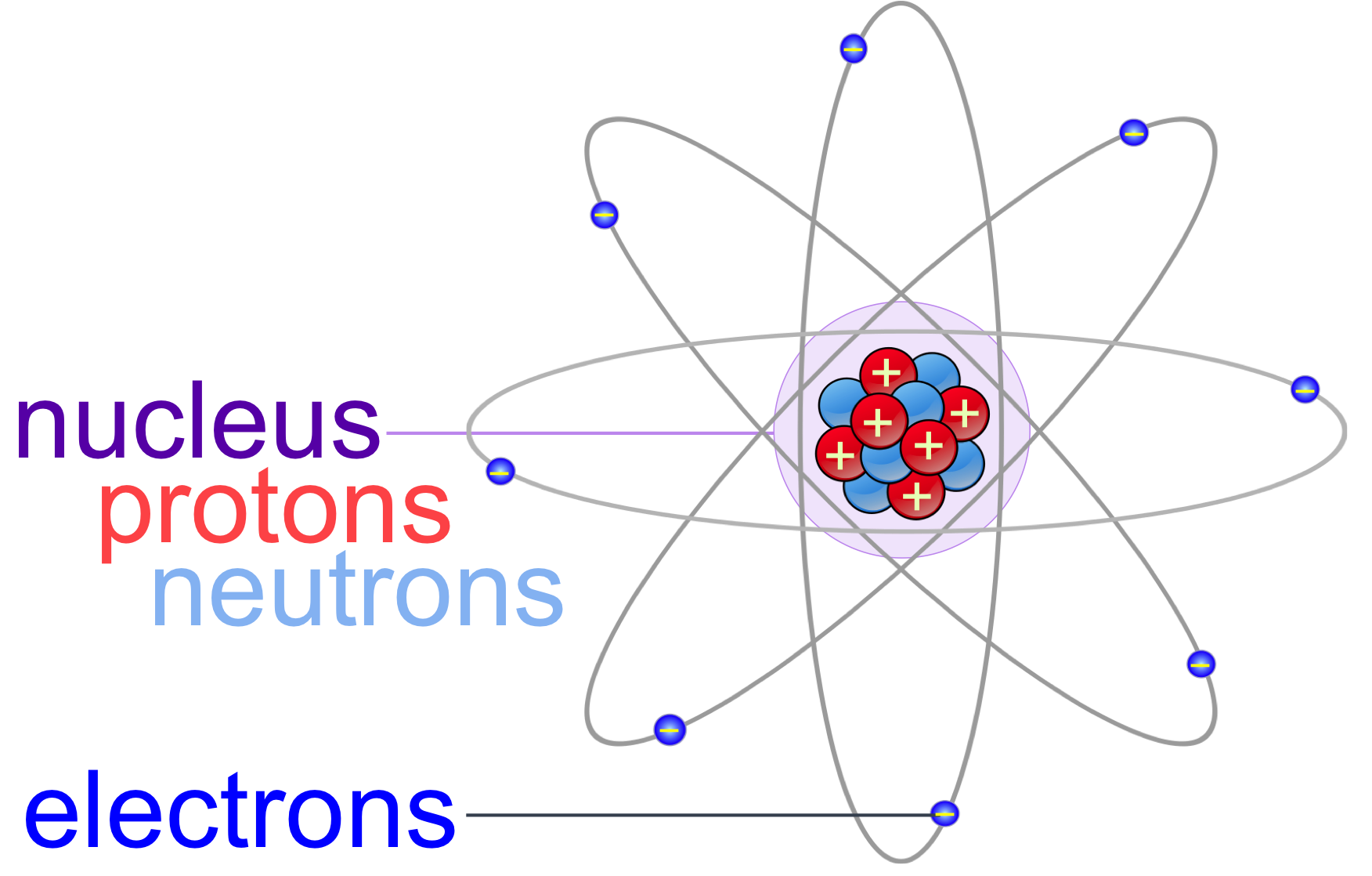
The electrons are found outside the nucleus. For nitrogen, the nucleus would look something like this: All of your atom's protons and neutrons go in the nucleus. The nucleus, the central part of the atom, is made from protons and neutrons. It is also helpful if the electrons are smaller than the protons and neutrons. It is helpful if the balls are color coded so that it is easier to tell which balls are protons, which are neutrons and which are electrons. Basically, anything that is roundish and that can be glued to each other should work. Ping-pong balls, rubber balls, ball bearings, golf balls and styrofoam balls have all been used in the past. Now that you know how many protons, neutrons and electrons you will need for your model, it is time to decide what to use to represent them. Using information found on the Periodic Table of Elements, we can tell that an average atom of nitrogen contains 7 protons, 7 neutrons and 7 electrons.
SIMPLE DIAGRAMS OF ATOMS HOW TO
If you do not already know how to use the Periodic Table of Elements to find this information, read the ' How many protons, electrons and neutrons are in an atom of.?' page to learn how. The barium cation is written Ba 2+, not Ba +2.Do you need to make a model or a drawing of an atom for science class? If so, follow these instructions to learn where all of the atom's pieces go.īefore you can build your model, you will need to know how many protons, neutrons and electrons your atom has. Note the convention of first writing the number and then the sign on a multiply charged ion. Figure 3.3 "Predicting Ionic Charges" shows how the charge on many ions can be predicted by the location of an element on the periodic table. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge.

:max_bytes(150000):strip_icc()/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Thus, the periodic table becomes a tool for remembering the charges on many ions. In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. In macroscopic samples of sodium chloride, there are billions and billions of sodium and chloride ions, although there is always the same number of cations and anions.
The number of electrons lost by the sodium atom (one) equals the number of electrons gained by the chlorine atom (one), so the compound is electrically neutral. Notice that there are no leftover electrons. The resulting combination is the compound sodium chloride. With two oppositely charged ions, there is an electrostatic attraction between them because opposite charges attract. On the right, the chloride ion has 18 electrons and has a 1− charge. On the left, the chlorine atom has 17 electrons. Most nonmetals become anions when they make ionic compounds.įigure 3.2 The Formation of a Chlorine Ion

Negatively charged ions are called anions A negatively charged ion. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Most metals become cations when they make ionic compounds. Positively charged ions are called cations A positively charged ion. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Most atoms do not have eight electrons in their valence electron shell. Use Lewis diagrams to illustrate ion formation.


 0 kommentar(er)
0 kommentar(er)
